Why We Can't Wave the White Flag on Early AF Detection
The jury is still out on whether wide-scale screening for atrial fibrillation (AF) in the general population is recommendable, considering the costs and complexity such workflows would entail. Data to support efficacy have thus far been mixed. Some studies have suggested that frequent opportunistic screening of high-risk patients, such as the elderly, in an outpatient setting, could increase diagnostic yield up to 5x compared to a single 12-lead ECG assessment.1 Other studies that analyzed similar cohorts in similar settings using point-of-care assessments showed no significant increase in AF detection rate or usefulness.2,3
Yet, we know that atrial fibrillation is still the most common heart rhythm disorder and inflicts significant morbidity and mortality.4 And it is going to get worse. As our population ages, the burden of AF will grow exponentially, with more than 12 million Americans predicted to have the disease by 2030.5 This means that we should redouble our efforts to catch AF and treat it early, by finding ways that are more cost-effective and more clinically sensitive. And if this glimpse at the future alone isn't enough to sound the alarm, here are five reasons we can't wave the white flag on developing early detection strategies.
The Incidence of Undiagnosed AF Could be as High as 40%
Most patients with AF will have episodes often enough to be detected in a doctor's office or overnight using a 24-hour monitor. However, about 1 in 4 will have sporadic ("paroxysmal") episodes that occur at unpredictable times.6 This means that doctors must rely instead on patients reporting symptoms such as shortness of breath, dizziness, or chest pain before they would even entertain further investigation. And what if those paroxysms go without symptoms? Then the physician must suspect AF from other history and physical exam findings and order the correct tests, which themselves have no guarantee of making a diagnosis because of the intermittent nature of the disease. Or they will be subjected to an odyssey of repeated visits and outpatient monitoring without a definitive diagnosis. All of this means that for perhaps as much as 40% of patients with AF, their disease will likely be missed. They will be walking around with undetected, untreated atrial fibrillation, waiting for a complication to bring them to medical attention.7
So, what can be done? Should we "look harder"? Or "look longer"? Studies that took a more continuous approach to opportunistic screening have indeed seen positive results. A 2020 study examining the diagnostic yield of AF screening in high-risk patients using a single-lead ECG in an outpatient setting showed that frequent examinations could lead to a 5-fold increase in detection compared to usual care.1
Another recent study analyzing a larger cohort using a continuous, wearable ECG patch saw a significantly increased rate of new AF detection for the actively monitored group.8 This study also demonstrated that it was feasible to implement a targeted AF detection strategy for high-risk patients without relying on fixed geographical sites, expanding potential reach to areas without specialty clinics.
No one would argue against the value of finding these currently undiagnosed AF patients. But with increased screening intensity comes increased cost, complexity, and burden on the patient, the clinician, and the health care system. And it raises the question of whether every patient with even the smallest burst of AF needs to be treated. Perhaps more intense screening is moving in the wrong direction, and instead, we should concentrate on making spot-screening tools more effective, cheaper, and more available?
Over 1 in 5 Patients with AF Suffers a Stroke Before Their AF is Diagnosed
For patients lucky enough to survive in the first place, the lifelong complications of a stroke can be debilitating, making prevention of utmost importance. Yet, one study showed that for nearly 22% of patients hospitalized from an ischemic stroke, AF was only diagnosed after the event occurred.9 And the problem was even more acute for younger patients, with over one-third of new AF cases diagnosed after hospitalization in patients below 75 years of age! This tells us that too many undiagnosed cases are still slipping by routine care and that a large percentage of patients are suffering downstream consequences of AF before the condition is diagnosed.
This is the scourge of silent AF, and it is frighteningly common. The study cited above also found that 30 to 40 percent of atrial fibrillation could be asymptomatic,9 which means that providers can't rely on patients bringing AF symptoms to their attention in a timely manner. What is even more worrisome is that AF may very frequently be the cause of "cryptogenic" strokes, that is, strokes without any apparent cause even after extensive investigation. One study found evidence that previously undiagnosed AF could be found in 12.4% of cases of cryptogenic stroke after a 12-month follow-up.10

Undiagnosed and Undertreated AF Adds Billions to Medical Spending
While cost is a major obstacle for targeted screenings using 12-lead ECGs, the financial burden of waiting until it's too late is also high. It is obvious that severe health consequences such as stroke are detrimental to patients' livelihoods, but there are also additional costs associated with AF.
One study looking at the economic impact of AF in the US estimated that the incremental medical costs of undiagnosed, non-valvular AF exceeds $3 billion annually.11 And those costs were not attributed only to stroke and its morbidity, but also included loss of work due to symptoms such as malaise, day-of-surgery cancelations due to sudden AF "discoveries", fatigue, and feelings of "impending doom," visits to urgent care settings, and admissions to the hospital for treatment. These financial findings suggest a strong economic benefit from catching and treating undiagnosed AF.
AF is Very Treatable if Caught Early
Despite all this doom and gloom, the good news is that the risks from AF can be drastically reduced if it is caught and treated early. Sometimes this means restoring a regular, normal sinus rhythm, and other times it means establishing anticoagulation. Restoring a normal rhythm achieves significant improvement in symptoms and returning to a normal lifestyle.12–14 Timely initiation of anticoagulation reduces stroke risk by as much as 64%, provided that the regimen is prescribed and followed properly.15
Even patients with infrequent episodes can benefit from early detection. Studies have shown that stroke risk for paroxysmal AF patients can vary greatly based on the collective time each individual spends in AF over a given period. One study found that patients with an AF burden of 11.4% or higher had an over 3-fold increased risk for stroke than those with a burden below 2%.16 Furthermore, this association was independent of other risk factors such as CHA2DS2-VASc and ATRIA scores. More individualized understanding of stroke risk will not only allow clinicians to make more precise determinations for care but keep patients informed and understanding of their own risk level to further incentivize adherence to their therapy.
Cost-effective Early Detection Tools Are Now Widely Available
With the abundance of digital technology available today, we now have far more options for designing and implementing systematic AF screening programs that are cost-effective, adaptable, and scalable. The issue isn't whether screening is worthwhile but whether it can become cost-effective enough to be implemented with adequate frequency. We should take what we've learned from previous studies and design new approaches that address these challenges. For example, opportunistic screening conducted at a single point in time will likely miss a large portion of paroxysmal and asymptomatic AF, and so we very likely need to screen more often.
This approach will need to be easy and cost-effective enough for patients and providers to apply regularly over a long period of time. For example, a study conducted on more than 7000 elderly patients in Sweden found that serial ECG screenings over a 2-week period yielded a 4-fold increase in detection compared to a single assessment.17 But rather than doing that screening with expensive 12-lead ECG machines that require specialized skill to administer, we can now deploy a variety of consumer ECG devices that are convenient to use and a fraction of the cost.18,19
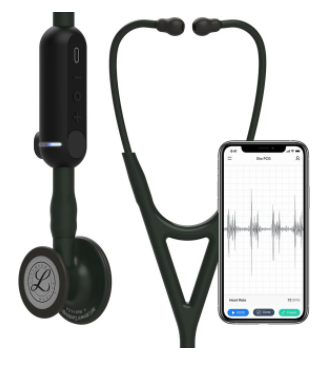
An added benefit of these inexpensive, software-enabled, patient-friendly rhythm monitoring devices is that they will become more valuable over time as the software and hardware improve. For example, one point-of-care cardiac assessment tool, the Eko DUO, allows for frequent, low-cost capture of heart rhythm and sounds in an outpatient or home care setting. Since it serves as the physician's stethoscope, it would be applied to every patient that the physician examines.
This means a device costing only a few hundred dollars that can screen for AF with 99% sensitivity, when used on thousands of patients per year, per physician, can drop the cost per screening to pennies. And because the DUO is a platform for machine learning software algorithms, it will only continue to improve: Currently, the DUO can detect AF and rapid or slow rhythms, but soon it will also be able to detect problems with the heart's pumping function and other abnormalities.
Low profile, convenient, and digitally augmented tools like the DUO can help providers shift efforts targeting AF detection from "not cost-effective" to "very cost-effective." They will be able to perform frequent ECG assessments during physical and virtual encounters that are faster, less cumbersome, and cheaper than using a 12-lead or ordering an ambulatory monitor.
This can be particularly impactful for primary and geriatric care professionals that frequently encounter older patients who are at high risk for AF or stroke. It's likely that low-cost and convenient devices like DUO could eventually serve as monitoring devices over the longer term, helping physicians keep track of their patients who are on drug therapies for AF or recovering from procedures such as heart surgery where AF is an extremely common and dangerous complication.
Additionally, for health care professionals who aren't performing ECG assessments regularly, being able to listen for rhythm irregularities using their stethoscope is another crucial opportunity to catch early signs of disease. Digitally augmented tools like the 3M Littmann CORE Digital Stethoscope help clinicians hear heart sounds more clearly. By using a unique combination of sound amplification and active noise cancellation, clinicians can catch even the subtlest sounds of irregular heart beats.
So, where do we go from here? We must generate evidence that screening patients with these devices is truly cost-effective and has a positive impact on patients and health care. That evidence will move the needle toward adopting AF screening as the standard of care and help reduce the burden of this terrible disease.
This article was originally posted on ekohealth.com as seen here.
References
- Zwart LA, Jansen RW, Ruiter JH, Germans T, Simsek S, Hemels ME. Opportunistic screening for atrial fibrillation with a single lead device in geriatric patients. J Geriatr Cardiol JGC. 2020;17(3):149-154. doi:10.11909/j.issn.1671-5411.2020.03.007
- Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, et al. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ. 2020;370:m3208. doi:10.1136/bmj.m3208
- Khurshid S, Li X, Ashburner JM, et al. Usefulness of Rhythm Monitoring Following Acute Ischemic Stroke. Am J Cardiol. 2021;147:44-51. doi:10.1016/j.amjcard.2021.01.038
- Sussman M, Di Fusco M, Tao CY, et al. The IMPact of UntReated NOn-Valvular Atrial Fibrillation on Short-TErm clinical and economic outcomes in the US Medicare population: the IMPROVE-AF model. J Med Econ. Published online August 20, 2021:1. doi:10.1080/13696998.2021.1970954
- CDC. Atrial Fibrillation | cdc.gov. Centers for Disease Control and Prevention. Published September 8, 2020. Accessed September 1, 2021. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213-220. doi:10.2147/CLEP.S47385
- Reiffel JA, Verma A, Kowey PR, et al. Incidence of Previously Undiagnosed Atrial Fibrillation Using Insertable Cardiac Monitors in a High-Risk Population: The REVEAL AF Study. JAMA Cardiol. 2017;2(10):1120-1127. doi:10.1001/jamacardio.2017.3180
- mSToPS: Active Screening For AFib Using ECG Patch Associated With Significant Improvement in Outcomes at Three Years. American College of Cardiology. Accessed September 1, 2021. https://www.acc.org/latest-in-cardiology/articles/2020/11/12/20/28/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f11%2f12%2f20%2f28%2fmon-1021am-mstops-aha-2020
- Jaakkola J, Mustonen P, Kiviniemi T, et al. Stroke as the First Manifestation of Atrial Fibrillation. PLoS ONE. 2016;11(12):e0168010. doi:10.1371/journal.pone.0168010
- Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. doi:10.1056/NEJMoa1313600
- Turakhia MP, Shafrin J, Bognar K, et al. Economic Burden of Undiagnosed Nonvalvular Atrial Fibrillation in the United States. Am J Cardiol. 2015;116(5):733-739. doi:10.1016/j.amjcard.2015.05.045
- Willems S, Borof K, Brandes A, et al. Systematic, early rhythm control strategy for atrial fibrillation in patients with or without symptoms: the EAST-AFNET 4 trial. Eur Heart J. Published online August 27, 2021:ehab593. doi:10.1093/eurheartj/ehab593
- Dossett ML, Needles EW, Donahue Z, et al. A SMART approach to reducing paroxysmal atrial fibrillation symptoms: Results from a pilot randomized controlled trial. Heart Rhythm O2. 2021;2(4):326-332. doi:10.1016/j.hroo.2021.06.003
- Martín A, Coll-Vinent B, Suero C, et al. Benefits of Rhythm Control and Rate Control in Recent-onset Atrial Fibrillation: The HERMES-AF Study. Acad Emerg Med Off J Soc Acad Emerg Med. 2019;26(9):1034-1043. doi:10.1111/acem.13703
- Lowres N, Giskes K, Hespe C, Freedman B. Reducing Stroke Risk in Atrial Fibrillation: Adherence to Guidelines Has Improved, but Patient Persistence with Anticoagulant Therapy Remains Suboptimal. Korean Circ J. 2019;49(10):883-907. doi:10.4070/kcj.2019.0234
- Go AS, Reynolds K, Yang J, et al. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018;3(7):601-608. doi:10.1001/jamacardio.2018.1176
- Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass Screening for Untreated Atrial Fibrillation. Circulation. 2015;131(25):2176-2184. doi:10.1161/CIRCULATIONAHA.114.014343
- Insider. ECG Apple Watch App: Monitor Your Heart Health. Accessed September 2, 2021. https://www.businessinsider.com/ecg-apple-watch
- Raja JM, Elsakr C, Roman S, et al. Apple Watch, Wearables, and Heart Rhythm: where do we stand? Ann Transl Med. 2019;7(17):417. doi:10.21037/atm.2019.06.79

